Thyroid cancer (TC) is the most common endocrine-related malignancy. It is projected that by 2030, it will become the fourth leading cancer diagnosis. Several known risk factors for TC include gender, race, head and neck irradiation, iodine deficiency, autoimmune thyroid disease, genetic risks, etc. The roles of other potential risk factors are still being investigated.
To find the possible trends and causes, The University of Nebraska Medical Center established a Thyroid Cancer and Tumour Collaborative Registry (TCCR). Using this registry, the researchers aim to assess and analyze the significance of suspected risk factors. This paper describes the operating procedures of TCCR and their best practices for data collection, sharing, and interoperability.
UNMC uses OpenSpecimen as their Biobanking LIMS and to annotate biospecimens obtained from the TCCR subjects
What is TCCR?
TCCR is a multicenter, web-based registry. The registry is open to any organization willing to adopt the TCCR standard questionnaire for data collection. Its mission is to provide a collaborative framework and standardized data and annotated biospecimens to support TC researchers.
In 2008, UNMC developed the first version of the TCCR, which utilized traditional multitier web architecture. Since then, the best practices for data collection, management, and sharing have been significantly advanced. Their goal is to improve the maintainability and sustainability of the TCCR, provide a foundation for its interoperability with other data sources, and make the TCCR more attractive for collaborative research.
To achieve this goal, they –
- Improved the questionnaire and workflow based on feedback from clinicians, epidemiologists, and the end-users
- Annotated the TCCR’s common data elements
- Harmonized these data elements with the NCI’s Cancer Data Standards Registry and Repository (caDSR); and
- Reengineered the TCCR using novel metadata-driven software architecture
Questionnaire and Common Data Elements
The target group for data collection includes adults of age group 19 and above who are diagnosed with TC/TNs and can give informed consent.
The time point of data collection include –
- Time of diagnosis
- 3, 6, and 12 months after diagnosis
- After that, annual visits
Patient information such as personal, demographic, medical, and family history is provided and entered by the participant. The clinical data such as diagnosis, surgery details are collected by the clinic personnel.
The questionnaire has been divided into two parts – Primary/Core and Extended/Optional. All data elements are annotated using the standard vocabularies such as SNOMED CT and the NCI Thesaurus and are harmonized with the NCI’s caDSR.
TCCR Operating Procedures
The TCCR Steering Committee oversees all the studies using TCCR data. The data collected at any center can be used by other registry users, but only after obtaining required permissions and upon agreement to provide appropriate references and acknowledgments.
The collaborators and representatives of TCCR have developed the standard organizational and operating procedures, and the security has been addressed using the electronic information security standards mandated by HIPAA.
The TCCR is accessible to registered users only. Authorized users are issued a unique electronic signature – a combination of the user name and a password. Also, each user has an appropriate level of access to data.
The data-sharing strategy is implemented in the following way:
- All data released to researchers is either de-identified or within the limited data set
- Data is used only if approved by the TCCR Steering Committee and IRB
- End-users are required to register and accept a license agreement during the registration; and
- While de-identified data are available to all registered users, PHI within the limited data set is provided only to users who sign a data use agreement.
Data Collection
Before starting data collection, the participating centers are required to obtain approval from their respective IRB. All participating researchers and clinicians must complete the computer-based training course on the Protection of Human Research Subjects.
To enter data, a copy of the subjects’ consent form must be submitted to the TCCR. If the participant withdraws consent, they may no longer participate in the research studies, and the use or sharing of future PHI will be stopped, but the PHI which has already been shared may still be used.
TCCR and OpenSpecimen
Many subjects enrolled in the TCCR agree to donate blood and urine samples and release a portion of tissue from a previous biopsy or surgical excision. The TCCR utilizes OpenSpecimen to track the collection, storage, and distribution of specimens. Biospecimen data collected by each participating center can be either submitted into the central repository or stored locally if a center maintains its installation of the OpenSpecimen.
The TCCR subject ID links the biospecimen data with the subject’s other data collected in the TCCR database. When a subject is registered in the TCCR, a corresponding record is automatically created in the OpenSpecimen to make it ready for biospecimen data collection.
Integration with OpenSpecimen significantly expanded the registry's capabilities, allowing researchers to manage and to mine biospecimen data for subjects enrolled in the TCCR.
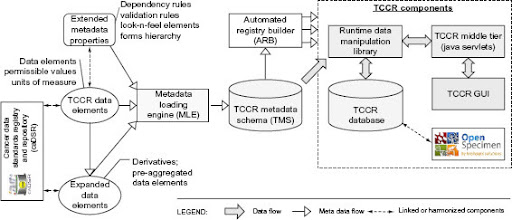
Architecture Diagram of TCCR
Conclusion
TCCR is a multicenter, web-based Thyroid Cancer and Tumor Collaborative Registry that collects and manages various data on thyroid cancer (TC) and thyroid nodule (TN) patients. The TCCR is coupled with OpenSpecimen, an open-source biobank management system, to annotate biospecimens obtained from the TCCR subjects. The TCCR is accessible to registered users only. While de-identified data are available to all registered users, PHI within the limited data set is provided only to users who sign a data use agreement.
Reference: https://journals.sagepub.com/doi/10.4137/CIN.S32470
