The first molecular tests for COVID-19 were ready for action in Europe a little over a month from the declaration of the pandemic. Similarly, the timeline for the more complex task of developing high-quality rapid antigen tests was compressed into just 8 months in comparison, the development of the first rapid test for HIV took 5 years. Even so, from early on in the pandemic, a critical bottleneck in the development of reliable diagnostic tests was the scarcity of clinical samples for diagnostic research, development, and validation.
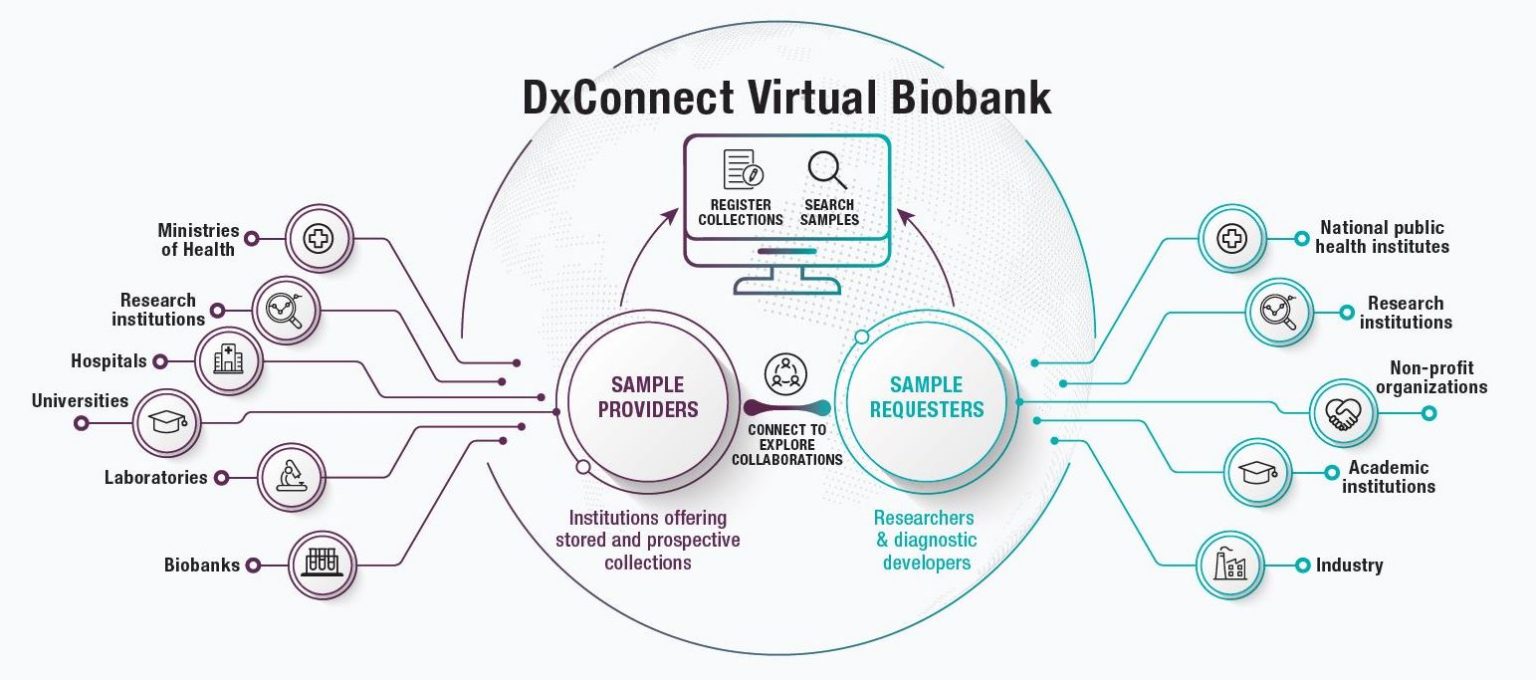
As pointed out in your June 2020 editorial, not only was there a shortage of specimens available for research but scientific progress was hampered by the complexity of both negotiating legal agreements for sample transfer and jurisdictional regimes regulating sample transport across borders. Read this article that describes DxConnect Virtual Biobank, which acts as a clearinghouse for specimens, connecting researchers with institutions around the world that have clinical samples available for the development and validation of new diagnostic tests. Set up and managed by FIND, it is one of the only free, open-access databases of its kind, and it is an important step in streamlining the development of diagnostic solutions.
Click here to read more.
