Managing biospecimen data for longitudinal studies or clinical trials can be challenging. Clinical studies often go on for months or years, and involves complex workflows like kit preparations, tracking upcoming visits, multi-site collections, shipments, and so forth. To manage these types of collections, OpenSpecimen offers a customizable platform to plan, manage and track biospecimens in a clinical study from collection to utilization.
OpenSpecimen is used by many centers as their primary biobanking LIMS to manage specimen collection for their clinical studies or trials. Some examples:
- Washington University uses OpenSpecimen to manage specimens for a large multi-site (multi-country) study called “The Alliance for Clinical Trials in Oncology”.
- FIND Diagnostics (Geneva) runs multiple clinical trials across Africa and Asia for infectious diseases like HIV, Malaria, etc.
- Nurture Biobank (UK) collects kidney specimens from 18 NHS hospitals and stores in a central location at the University of Bristol.
Key Highlights
- Design protocols with time points and the specimens expected per time point
- Define specimen processing steps (For example, process whole blood into plasma and PBMC, and create aliquots and store)
- Configure label formats for patient IDs and specimen labels/barcodes
- Create different roles per clinical coordinators
- Set different strategies for container auto allocation(For example, choose box based on study and type of specimens)
- Track shipments from multiple sites to the central repository
- Configure specimen label printing (including pre-printing for kit preparations)
- Integrate with EDC (E.g. REDCap, OpenClinica, etc) or CTMS (e.g. OnCore, Medidata Rave, etc)
- Design graphical dashboards and reports to track collections across time points
Designing a Protocol
You can create a specimen collection calendar with time points such as ‘baseline’, ‘pre-operative’, ‘post-operative’ etc. With each time point, you can define the specimens to be collected and processed along with their properties such as type and quantity.
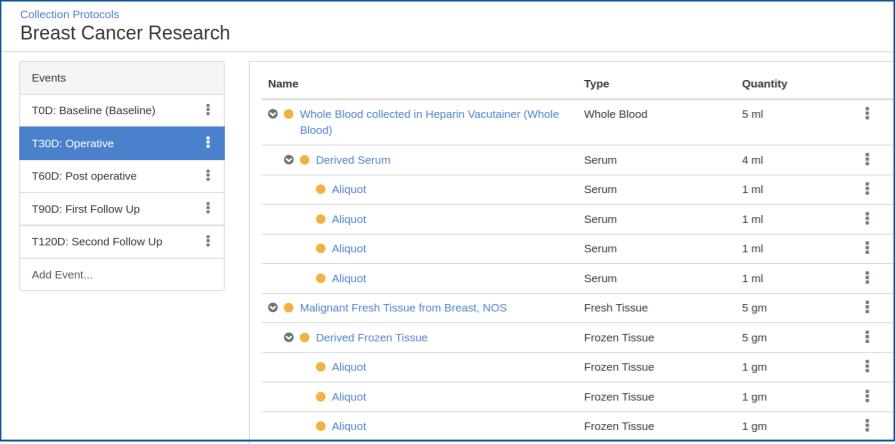
Data Management
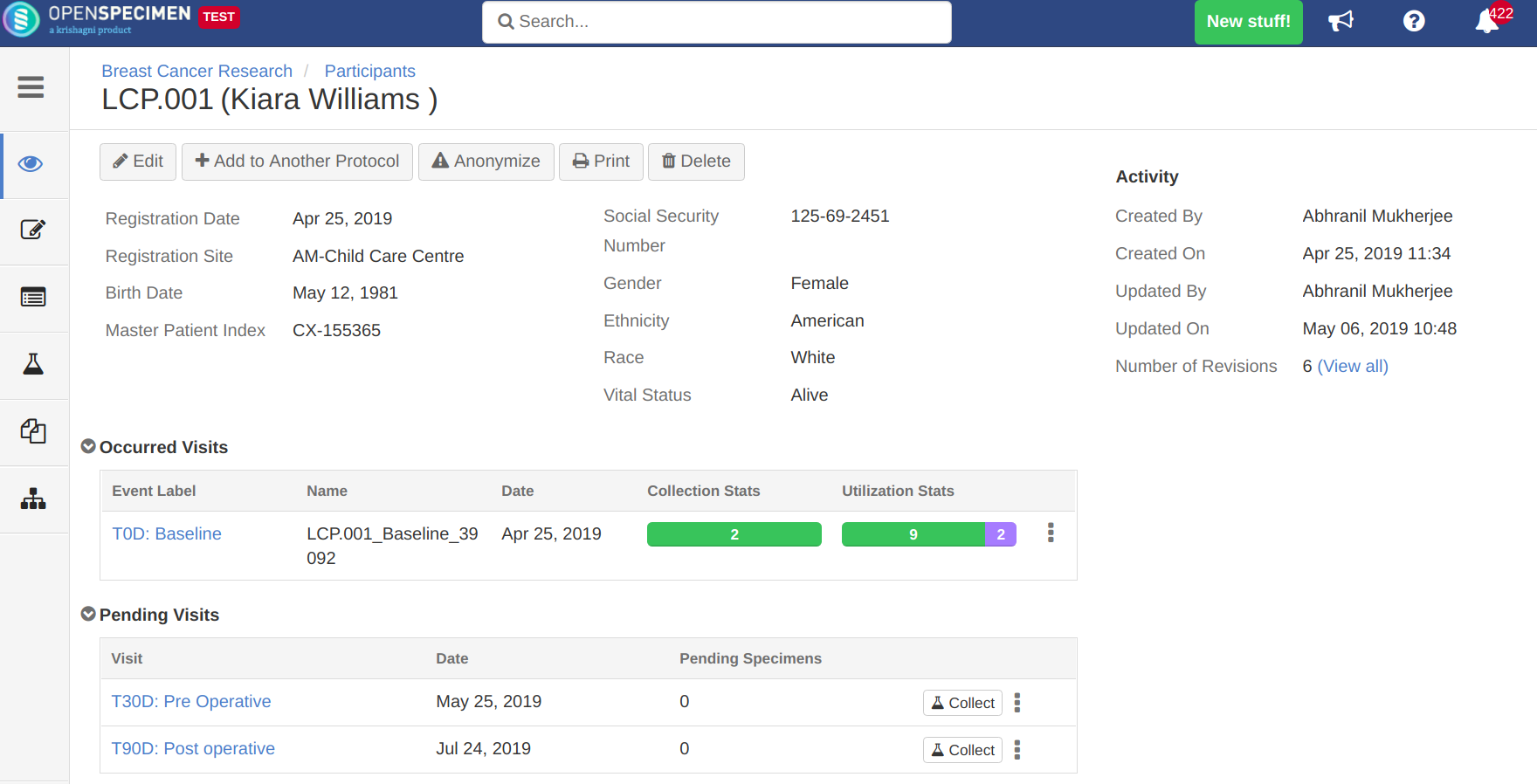
The following data can be collected per participant:
- Participant registration and consents
- Additional questionnaires (e.g. smoking history, medical history)
- Visits and visit level forms (e.g. pathology annotations)
- Specimens and any associated data
The specimens can be collected by the Clinician role users as per the study definition. After collection, specimens can be shipped to a biorepository and processed further. Any deviations in collections or processing (like extra specimens or lower quantity) can be handled on the fly.
Auto Labeling and Printing
OpenSpecimen can be configured to auto-generate and print specimen labels with a format of your choice. E.g. Study Code, Participant Code, Specimen Abbreviation, Unique Sequence, etc.
You can also pre-print the labels for upcoming visits.
Container Auto-allocation
In clinical studies, commonly the specimen types and their storage are well defined. In such cases, OpenSpecimen can be configured to automatically suggest the next relevant box to store the specimen based on allocation rules defined.
Reports
Reporting is one of the most powerful features of OpenSpecimen. Without any IT support, you can create reports to get raw data or counts based summary reports. E.g. count of specimens collected per site by specimen type, list of specimens by box, specimens expected to be collected next week, etc. Reports can be saved, shared, scheduled, etc.
Summary
OpenSpecimen provides a platform for designing longitudinal study templates and managing participant visit records. Additionally, it allows specimen labeling and storage. Tracking the progress of the study or generating customized reports becomes easy with the query tool of OpenSpecimen.
Customer Speak
“OpenSpecimen is used in combination with the Alliance BioSpecimen Management System (BioMS). The integration enables real-time data synchronization so that new subjects registered in BioMS are automatically registered in OpenSpecimen – thereby reducing manual data entry and eliminating errors due to duplication”
–Washington University (St. Louis).
“We use OpenSpecimen to track sample use and to find specimens for UC Davis researchers. With its extensive configurability and ability to create whatever custom data entry forms are needed, OpenSpecimen is just perfect for us.”
–University of California, Davis.
The integration of OpenSpecimen with OpenClinical (EDC) has improved tracking and minimization of human errors. This improved the quality of data and help in meeting the QA/QC standards.
